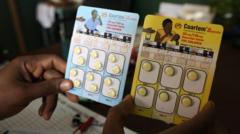
The first malaria treatment designed specifically for babies and young children has received approval, with plans for rollout in African nations soon. Historically, no approved treatments existed for infants; they were administered medications created for older children, increasing the risk of overdose. In 2023, malaria resulted in approximately 597,000 deaths, primarily in Africa, with three-quarters of these fatalities among children under five.
Novartis developed the new drug, which is expected to be introduced primarily on a not-for-profit basis. The treatment, referred to as Coartem Baby, offers an optimized dosage that ensures safety for the most vulnerable patients. The drug was created in collaboration with the Medicines for Malaria Venture (MMV) and has been evaluated through trials in eight African countries.
Experts emphasize the significance of this approval in combating malaria, particularly since over 76% of-related deaths affect children in regions like sub-Saharan Africa. The hope is that with the right resources and attention, the impact of malaria can be diminished, paving the way towards improved healthcare accessibility for those in need.