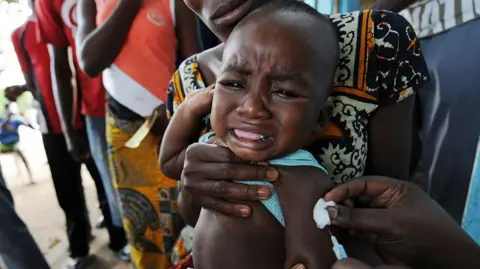
A now-halted plan to run a hepatitis B vaccine trial involving thousands of newborns in Guinea-Bissau has been criticized by the World Health Organization (WHO) as 'unethical'. The US-funded study had sought to give one set of babies the vaccine at birth, while another would have had the shot delayed until six weeks of age.
Who expressed 'significant concerns' about the study, highlighting that the birth-dose vaccine is an effective public health intervention with a proven record. The US health department, led by Robert F Kennedy Jr., who has been skeptical about vaccines, proposed this trial to explore the vaccine's broader health effects.
The WHO emphasized that the trial's setup—offering a life-saving intervention to some while denying it to others—could lead to irreversible harm. Vaccination at birth has shown to prevent hepatitis B transmission from mother to child in 70-95% of cases. With more than 12% of Guinea-Bissau's population living with chronic hepatitis B, the WHO argues that this trial exposes newborns to risk when they should receive the vaccine immediately after birth.
The trial's suspension came after public outrage, with critics, including former health minister Magda Robalo, stating that Guinea-Bissauans should not be treated like 'guinea pigs'. The WHO recommends that all newborns receive the hepatitis B vaccine within 24 hours of birth, yet currently, in Guinea-Bissau, it is administered at six weeks, with a nationwide rollout of the birth dose planned for 2028. As of now, the trial intended to involve 14,000 babies.
This controversy arises amidst broader discussions about vaccine policies, including recent changes in the US regarding hepatitis B vaccine recommendations.
Who expressed 'significant concerns' about the study, highlighting that the birth-dose vaccine is an effective public health intervention with a proven record. The US health department, led by Robert F Kennedy Jr., who has been skeptical about vaccines, proposed this trial to explore the vaccine's broader health effects.
The WHO emphasized that the trial's setup—offering a life-saving intervention to some while denying it to others—could lead to irreversible harm. Vaccination at birth has shown to prevent hepatitis B transmission from mother to child in 70-95% of cases. With more than 12% of Guinea-Bissau's population living with chronic hepatitis B, the WHO argues that this trial exposes newborns to risk when they should receive the vaccine immediately after birth.
The trial's suspension came after public outrage, with critics, including former health minister Magda Robalo, stating that Guinea-Bissauans should not be treated like 'guinea pigs'. The WHO recommends that all newborns receive the hepatitis B vaccine within 24 hours of birth, yet currently, in Guinea-Bissau, it is administered at six weeks, with a nationwide rollout of the birth dose planned for 2028. As of now, the trial intended to involve 14,000 babies.
This controversy arises amidst broader discussions about vaccine policies, including recent changes in the US regarding hepatitis B vaccine recommendations.